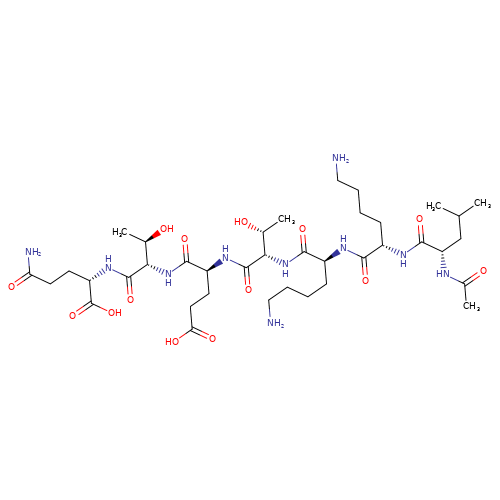
TB500 10mg vial
Hämtning är inte tillgänglig just nu
NOT FOR HUMAN CONSUMPTION
TB-500, also known as Thymosin Beta-4 (Tβ4), is a synthetic peptide derived from a naturally occurring protein found in virtually all human and animal cells. It plays a critical role in cell migration, wound healing, tissue regeneration, and reducing inflammation. Initially discovered in the thymus gland, Thymosin Beta-4 has garnered significant attention in clinical and sports research due to its regenerative and anti-inflammatory properties.
Biological and Chemical Properties
-
Name: Thymosin Beta-4 (Tβ4), commonly referred to as TB-500 (synthetic form)
-
Structure: 43-amino-acid peptide
-
Natural Source: Ubiquitously expressed in various tissues; particularly abundant in wound fluids and platelets
-
Administration Route: Subcutaneous or intramuscular injection for experimental purposes
-
Half-life: Approximately 24–48 hours (rapid tissue distribution with prolonged regenerative effects)
Mechanism of Action
TB-500 exerts its effects primarily through:
1. Enhanced Cell Migration and Healing
-
Promotes cell migration and adhesion, accelerating wound closure and tissue regeneration.
-
Influences actin polymerization, essential for cell motility and tissue repair.
2. Angiogenesis (Blood Vessel Formation)
-
Stimulates the growth of new blood vessels, improving blood flow, oxygenation, and nutrient delivery to injured tissues.
3. Anti-inflammatory Effects
-
Reduces inflammatory cytokines and modulates immune responses, facilitating a faster healing process and reduced scar tissue formation.
4. Tissue Protection and Regeneration
-
Exhibits protective effects on cardiac, neural, and muscular tissues, potentially mitigating injury-related damage.
Therapeutic and Experimental Applications
TB-500 demonstrates substantial therapeutic potential across multiple conditions:
1. Musculoskeletal Injuries and Recovery
-
Accelerates healing in muscle, tendon, ligament, and joint injuries.
-
Popular among athletes and bodybuilders experimentally to speed recovery from soft tissue injuries.
2. Cardiovascular Disease
-
Cardioprotective properties following myocardial infarction (heart attack), reducing damage and aiding cardiac repair (animal and preliminary human studies).
3. Wound Healing and Skin Repair
-
Significant improvement in wound closure rates, reduced scar formation, and enhanced skin healing in preclinical models.
4. Neuroprotection and Nervous System Regeneration
-
Preclinical evidence of neuroprotective benefits in traumatic brain injury (TBI), stroke, and peripheral nerve regeneration.
5. Inflammatory and Autoimmune Conditions
-
Experimental potential in treating inflammatory disorders, reducing symptoms, and improving recovery rates.
Dosage and Administration (Experimental Context)
No standardized clinical guidelines exist, as TB-500 remains experimental. Common dosing strategies used in experimental and anecdotal contexts include:
| Context | Typical Experimental Dose | Administration Frequency |
|---|---|---|
| Soft Tissue Injury and Recovery | 2–4 mg per injection | 1–2 times weekly for 4–6 weeks |
| Maintenance/Chronic Injuries | 2 mg per injection | Once weekly |
| Severe Injuries (Experimental) | Up to 5 mg initially | Twice weekly for initial 2 weeks |
Administration: Usually subcutaneous injection near injury site or general subcutaneous injection.
Safety Profile and Side Effects
TB-500 is generally reported as well-tolerated in limited experimental use. However, due to limited clinical studies, complete safety profiles are unknown.
Commonly Reported (Rare and Mild):
-
Mild transient irritation or redness at injection sites.
-
Temporary fatigue or headache (rarely reported).
Potential Long-term Risks:
-
Limited data on long-term safety in humans.
-
Theoretical concerns of promoting uncontrolled angiogenesis or tumor growth, though not confirmed in clinical studies.
Contraindications and Precautions
-
Contraindications:
-
Pregnancy and breastfeeding (unknown safety).
-
Known hypersensitivity or allergic reaction to peptides.
-
-
Precautions:
-
Individuals with active or prior malignancies (theoretical risk of angiogenesis).
-
Caution in individuals prone to autoimmune conditions or hyperactive immune responses.
-
Legal and Regulatory Status
-
Approval Status:
-
Not approved by FDA or other regulatory agencies for clinical therapeutic use in humans.
-
-
Availability:
-
Typically marketed as a research peptide "not for human consumption."
-
-
Sports and Doping:
-
Explicitly prohibited by World Anti-Doping Agency (WADA) due to regenerative and performance-enhancing potential.
-
Current Research Status and Evidence
-
Extensive preclinical (animal) studies confirming regenerative potential for muscle, tendon, ligament injuries, cardiovascular damage, and wound healing.
-
Limited human clinical studies have been conducted; early trials demonstrate safety and promising efficacy but require further investigation.
-
Most current human use remains experimental, anecdotal, or investigational, with ongoing research required for robust validation.
Summary of Potential Benefits and Risks
| Potential Benefits | Potential Risks and Limitations |
|---|---|
| Accelerated healing of musculoskeletal injuries | Lack of extensive long-term human safety data |
| Anti-inflammatory and reduced scarring effects | Theoretical concerns of angiogenesis and cancer promotion |
| Cardioprotective and neuroprotective properties | Regulatory uncertainty and lack of approved clinical guidelines |
| Potential therapy in chronic inflammatory disorders | Experimental status and limited clinical data |
Future Directions and Research Needs
-
Conduct large-scale, randomized, controlled human clinical trials to confirm efficacy, dosage optimization, and safety.
-
Long-term studies assessing safety, potential cancer risk, and impacts on immune function.
-
Expansion of therapeutic potential investigations into chronic inflammatory, cardiovascular, neurodegenerative, and musculoskeletal conditions.
References
-
Sosne, G., & Kleinman, H. K. (2015). "Thymosin Beta 4 promotes dermal healing." Expert Opinion on Biological Therapy, 15(Suppl 1), S47–S53.
-
Goldstein, A. L., & Hannappel, E. (2005). "Thymosin β4: actin-sequestering protein moonlights to repair injured tissues." Trends in Molecular Medicine, 11(9), 421–429.
-
Smart, N., Risebro, C. A., & Riley, P. R. (2007). "Thymosin β4 and cardiac repair." Annals of the New York Academy of Sciences, 1112, 82–91.