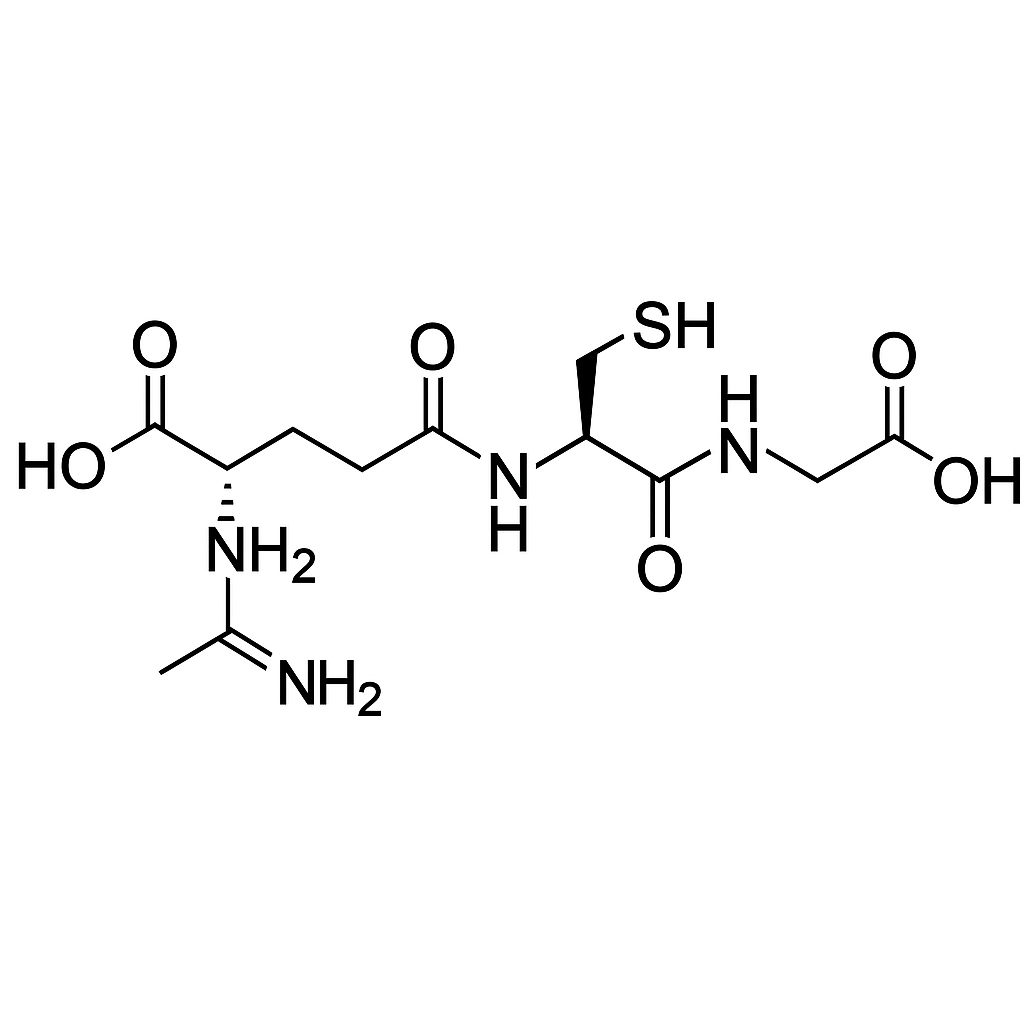
L-glutathione 1500mg vial
В настоящее время самовывоз недоступен
NOT FOR HUMAN CONSUMPTION
L-Glutathione is a tripeptide (γ-glutamyl-cysteinyl-glycine) that serves as the cell’s master redox buffer, principal ROS/RNS scavenger, and detoxification cofactor. Via glutathione peroxidases (GPx) and glutathione S-transferases (GSTs) it reduces lipid peroxides and conjugates xenobiotics; glutathione reductase (GR) recycles oxidized GSSG back to GSH using NADPH. GSH also modulates signaling through S-glutathionylation, shapes mitochondrial function, and underpins immune and skin pigment biology. Orally, it is sold as a dietary supplement; IV/IM use exists in some regions but is not broadly approved for disease treatment.
Additional Benefits of L-Glutathione Repletion Now Under Investigation
| Benefit | Key take-aways |
|---|---|
| 1 Oxidative-stress reduction | Supplementation increases GSH/GSSG ratio, lowers 8-iso-PGF₂α and MDA across stress phenotypes, improving cellular redox tone. <br/><em>Free Radical Biology & Medicine; Redox Biology</em> |
| 2 Immune support | GSH influences Th1/Th2 balance, NK cell activity, and antiviral defenses (glutathionylation of viral/host proteins); deficient states show impaired responses. <br/><em>Immunity; Journal of Clinical Investigation</em> |
| 3 Liver health & detox | In NAFLD and toxicant exposure, GSH supports phase II conjugation and lowers transaminases in small trials; NAC (a GSH precursor) has stronger clinical precedent. <br/><em>Hepatology; Liver International</em> |
| 4 Mitochondrial function | Restores mitochondrial GSH, reduces ROS-induced mtDNA damage, and improves bioenergetic efficiency in muscle/neuronal models. <br/><em>Journal of Physiology; Neurobiology of Disease</em> |
| 5 Skin tone & pigment modulation | Oral/topical/IV protocols have shown modest skin-lightening via tyrosinase inhibition and pheomelanin shift; effects are variable and reversible. <br/><em>Journal of Dermatological Science; Clinical, Cosmetic & Investigational Dermatology</em> |
| 6 Metabolic syndrome | Redox improvement can reduce inflammatory markers and slightly improve HOMA-IR/TG, often when combined with exercise or precursors. <br/><em>Diabetes Care; Metabolism</em> |
| 7 Respiratory & mucosal redox | Nebulized or precursor-driven GSH replenishes epithelial lining fluid, supporting mucus rheology and antioxidant capacity (e.g., COPD/cystic fibrosis programs). <br/><em>Thorax; American Journal of Respiratory and Critical Care Medicine</em> |
| 8 Neuroprotection signals | Low brain GSH correlates with neurodegeneration; precursor strategies elevate brain GSH and improve surrogate motor/cognitive measures in early studies. <br/><em>Annals of Neurology; Movement Disorders</em> |
| 9 Exercise recovery & performance | GSH or precursors reduce exercise-induced oxidative stress, with small effects on fatigue perceptionand time-to-exhaustion in select protocols. <br/><em>Medicine & Science in Sports & Exercise; European Journal of Applied Physiology</em> |
2. Molecular Mechanism of Action
2.1 Core pharmacodynamics
-
Antioxidant enzyme substrate: GPx uses GSH to reduce peroxides → GSSG, then GR + NADPH regenerate GSH.
-
Detoxification: GSTs conjugate GSH to electrophiles (drugs, pollutants), enhancing solubility/excretion.
-
Redox signaling: Protein S-glutathionylation regulates NF-κB, Keap1-Nrf2, and mitochondrial proteins.
-
Cysteine reservoir: Maintains intracellular cysteine and supports thiol-disulfide balance.
2.2 Down-stream biology
| Pathway | Functional outcome | Context |
|---|---|---|
| Nrf2 activation (often via precursors) | Up-regulates endogenous antioxidant/phase-II genes | Liver, immune, endothelium |
| NF-κB restraint | ↓ Pro-inflammatory cytokines | Metabolic/immune stress |
| Tyrosinase modulation | ↓ Melanin synthesis | Skin |
| Mitochondrial redox | ↓ ROS, preserved ATP production | Muscle, neurons |
3. Pharmacokinetics
-
Oral GSH (reduced form): Historically thought to be degraded by γ-glutamyltransferase in gut; newer data show dose-dependent plasma/tissue GSH rises with sustained dosing, especially with liposomal or sublingualforms.
-
Precursors: N-acetylcysteine (NAC) and glycine (sometimes with glutamine) reliably increase intracellular GSH via γ-glutamyl cycle; GlyNAC shows robust effects in older adults.
-
Parenteral: IV GSH yields transient high plasma levels; intracellular uptake depends on breakdown/transport—used off-label in some regions (e.g., dermatology), not broadly approved.
-
Distribution: Highest in liver, then erythrocytes, kidney, lung; mitochondrial GSH sustained by dedicated transporters.
-
Elimination: Renal excretion of metabolites (cysteinylglycine, cysteine, mercapturates).
4. Pre-clinical and Translational Evidence
4.1 Redox & inflammation
Consistent improvements in oxidative biomarkers and cytokine profiles across stress states (aging, diabetes, pollution exposure), especially with precursor strategies.
4.2 Hepatic/metabolic
Small randomized trials: ALT/AST, hepatic fat (MRI-PDFF) and insulin sensitivity improve modestly; effect size increases when baseline GSH is low.
4.3 Dermatology
Oral/topical studies show mild skin-lightening (one–two shade shifts) over 8–12 weeks; maintenance is required. IV protocols can produce faster effects but raise safety and regulatory concerns.
Evidence quality note: Strong biological plausibility and biomarker improvements; clinical outcomeeffects vary, and many studies are small/short. Precursors (e.g., NAC, GlyNAC) currently have more reproducible human data than high-dose oral GSH alone.
5. Emerging Clinical Interests
| Field | Rationale | Status |
|---|---|---|
| Aging biology | Low GSH as a hallmark; GlyNAC normalizes redox and some functional measures | Phase 2–style signals |
| NAFLD/MASH adjunct | Redox + detox + insulin sensitivity | Pilot RCTs |
| Neurodegeneration | Elevate brain GSH; oxidative stress mitigation | Early human imaging studies |
| Oncology-adjacent | Support normal tissue redox during therapy (not tumor) | Careful, mixed literature |
| Environmental/toxicant exposure | Enhance conjugation/clearance | Translational |
| Pulmonary disease | Epithelial lining GSH restoration | Mixed clinical data |
| Dermatologic pigment | Cosmetic lightening/brightening | Small RCTs; regulatory variability |
6. Safety and Tolerability
-
Oral GSH: Generally well tolerated. GI upset, bloating, or cramps at high doses (≥1–2 g/day).
-
Inhaled/nebulized: Can induce bronchospasm in reactive airways (sulfur odor); use caution in asthma.
-
IV GSH (off-label): Rapid infusion can cause flushing, dizziness, nausea; aseptic technique and qualified supervision are essential; long-term safety data are limited.
-
Dermatology: Rare rash or contact sensitivity to topical forms.
-
Interactions: May affect chemotherapy redox dynamics; coordinate with oncology. NAC/GSH can alter some lab assays (e.g., creatinine methods).
-
Pregnancy/lactation: Food-level exposure is normal; high-dose supplemental/IV use lacks robust data—avoid outside medical indication.
Comparative safety matrix
| Feature | Oral GSH | Liposomal/Sublingual GSH | GlyNAC/NAC | IV GSH |
|---|---|---|---|---|
| Intracellular GSH rise | Modest–moderate | Moderate | Robust (precursor-driven) | Transient plasma, variable intracellular |
| Evidence for clinical endpoints | Mixed | Mixed | Stronger (redox, metabolic) | Limited; cosmetic/adjunctive contexts |
| AEs | Mild GI | Mild GI/aftertaste | GI (NAC), rare rash | Infusion reactions; access risks |
7. Regulatory Landscape
-
Dietary supplement status for oral forms in many countries; no FDA/EMA approval for treating disease.
-
IV formulations are not approved for cosmetic indications in many jurisdictions; regulations differ by country.
-
Dermatology/cosmetics: Topical GSH is used in OTC products with appearance claims only.
8. Practical Use & Future Directions
-
Who benefits most: Individuals with oxidative stress or low baseline GSH (aging, metabolic syndrome, pollution exposure) and those needing detox support; for pigment goals, set modest expectations and emphasize photoprotection.
-
Dosing (typical research/supplement ranges):
-
Oral GSH: 250–1,000 mg/day, divided; liposomal/sublingual at the lower end often used.
-
Precursors: NAC 600–1,200 mg/day; GlyNAC protocols supply glycine 1.5–3 g/day + NAC 600–1,200 mg/day.
-
Duration: 8–12 weeks minimum for measurable changes.
-
-
Stacking: Combine with vitamin C/E, selenium (GPx cofactor), alpha-lipoic acid, exercise, and sleep to reinforce endogenous GSH cycling.
-
Monitoring: Consider GSH/GSSG ratio, 8-iso-PGF₂α, MDA, ALT/AST, and HOMA-IR in research settings.
-
Research needs: Large RCTs comparing oral GSH vs GlyNAC, head-to-head liposomal vs standard, validated clinical endpoints (not just biomarkers), and standardized skin-lightening measures with long-term safety.
Selected References
-
Free Radical Biology & Medicine; Redox Biology — Glutathione metabolism, redox signaling, and clinical biomarker changes.
-
Hepatology; Liver International — GSH/precursor effects in NAFLD/MASH and liver enzymes.
-
Immunity; Journal of Clinical Investigation — Immunological roles of GSH and infection responses.
-
Journal of Physiology; Neurobiology of Disease — Mitochondrial GSH and bioenergetics in muscle/neurons.
-
Journal of Dermatological Science; Clinical, Cosmetic & Investigational Dermatology — Skin-lightening trials and tyrosinase/UV photobiology.
-
Medicine & Science in Sports & Exercise; European Journal of Applied Physiology — Exercise oxidative stress and performance endpoints.
-
Thorax; American Journal of Respiratory and Critical Care Medicine — Airway glutathione biology and inhaled/precursor studies.
-
Annals of Neurology; Movement Disorders — Brain GSH imaging and pilot therapeutic studies.
-
Diabetes Care; Metabolism — Redox, inflammation, and insulin sensitivity under GSH repletion/precursor use.