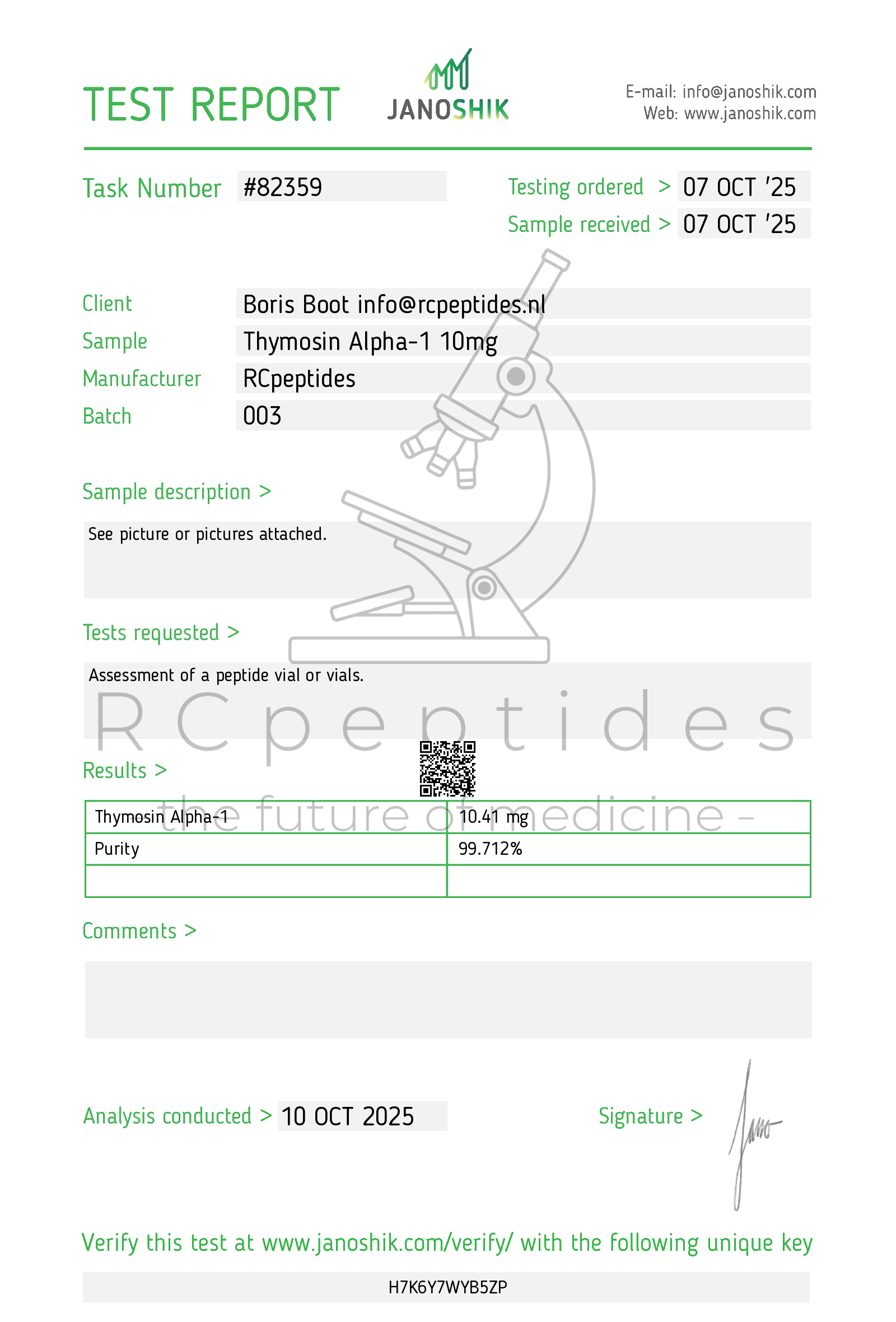
Thymosin alpha-1 10mg vial
Pickup currently not available
NOT FOR HUMAN CONSUMPTION
Thymosin α1 is a 28–amino-acid synthetic peptide originally isolated from thymic extracts (thymosin fraction 5). It acts as a host-directed immunomodulator, enhancing antigen presentation (DC maturation), T-cell priming (Th1 bias), NK-cell cytotoxicity, and re-balancing dysregulated cytokine networks. Mechanistically it interfaces with pattern-recognition pathways (e.g., TLR2/7/9 on dendritic cells) and intracellular NF-κB/IRF signalling, improving antiviral and antitumour responses while limiting hyper-inflammation. Thymosin α1 is approved in several countries (ex-US/EU)—historically for chronic hepatitis B/C and adjunctive immunotherapy—not FDA-approved in the United States.
Additional Benefits of Thymosin α1 Now Under Investigation
| Benefit | Key take-aways |
|---|---|
| 1 Antiviral host defence (HBV/HCV and beyond) | In chronic hepatitis, Tα1 improves HBeAg seroconversion and ALT normalization and augments interferon/NA responses; newer work explores HDV and difficult-to-cure HBV phenotypes. <br/><em>Hepatology; Journal of Hepatology</em> |
| 2 Vaccine adjuvancy | Peri-vaccination courses increase seroconversion rates and antibody titres in hypo-responders (elderly, CKD, cirrhosis) and enhance T-cell responses to viral and tumour antigens. <br/><em>Vaccine; Human Vaccines & Immunotherapeutics</em> |
| 3 Oncology (immunotherapy adjunct) | Tα1 boosts DC cross-presentation and CD8⁺ priming, showing synergy with checkpoint inhibitors and chemo-immunotherapy in early trials for melanoma, HCC, and lung cancer. <br/><em>Cancer Immunology Research; Annals of Oncology</em> |
| 4 Sepsis/critical-illness immunoparesis | In lymphopenic/septic phenotypes, Tα1 restores HLA-DR on monocytes, increases IFN-γ/IL-2while reducing IL-6/TNF-α, with signals for lower secondary infection and mortality in select cohorts. <br/><em>Critical Care; Intensive Care Medicine</em> |
| 5 Respiratory infections & ARDS (mixed evidence) | Observational and small RCTs suggest reduced progression in viral pneumonias when given to lymphopenic patients; other trials are neutral, underscoring the need for phenotype-guided use. <br/><em>Chest; The Lancet Respiratory Medicine</em> |
| 6 Immunosenescence & frailty | Short courses increase naïve/memory T-cell markers, TRECs, and NK cytotoxicity in older adults, with fewer seasonal infections and better vaccine responses. <br/><em>Gerontology; Mechanisms of Ageing and Development</em> |
| 7 Autoimmune “rebalance” (pilot) | By promoting Treg stability and dampening pathologic Th17/Th2 signals, Tα1 is being explored as an adjunct in autoimmune thyroid disease, IBD, and psoriasis (early-phase data). <br/><em>Clinical Immunology; Journal of Autoimmunity</em> |
| 8 Onco-hematology support | Adjunct Tα1 shortens neutropenia duration, improves infection control, and may speed count recovery with chemotherapy or transplant conditioning. <br/><em>Bone Marrow Transplantation; Supportive Care in Cancer</em> |
| 9 Metabolic-liver disease (NAFLD/NASH) | Proof-of-concept studies show ALT/AST and CAP/MRI-PDFF improvements with immune/metabolic re-tuning; larger controlled trials are pending. <br/><em>Hepatology Communications; Liver International</em> |
2. Molecular Mechanism of Action
2.1 Pharmacodynamics
Tα1 engages TLR2/7/9 (context-dependent) on dendritic cells and monocytes, promoting maturation (↑CD80/86, ↑HLA-DR) and IL-12 production; this licenses Th1 and CTL responses. In T cells, Tα1 enhances IL-2R (CD25)expression, IFN-γ production and survival signalling, while tempering excessive NF-κB activity in hyper-inflammatory states.
2.2 Down-stream Biology
| Pathway | Functional outcome | Context |
|---|---|---|
| TLR→MyD88→NF-κB/IRFs | DC maturation, type-1 cytokines, antiviral genes | Innate/APC compartments |
| IL-12/IFN-γ axis | Th1 polarization, CTL activation | Adaptive T/NK cells |
| Treg/Th17 balance | ↑ Treg stability, ↓ pathogenic Th17 | Autoimmunity/inflammation |
| Antigen presentation | ↑ HLA-DR, cross-presentation | DCs/monocytes |
| NK effector pathways | ↑ Perforin/Granzyme, cytotoxicity | NK cells |
3. Pharmacokinetics
-
Route: Subcutaneous is standard; IV used in some trials.
-
Absorption & t½: Rapid absorption; terminal half-life ~1.5–2.0 h (SC); pharmacodynamic effects outlast exposure via transcriptional re-programming.
-
Dosing paradigms (by indication/trials): 1.6 mg SC 2×/week (chronic hepatitis) for months; daily SC in short ICU/oncology courses; peri-vaccination short cycles.
-
Clearance: Peptidase degradation; no CYP interactions expected.
4. Pre-clinical and Translational Evidence
4.1 Chronic Viral Hepatitis
Multiple RCTs (legacy and modern) show improved biochemical and serologic responses with Tα1 alone or combined with interferon or nucleos(t)ide analogues; durability varies by genotype and baseline immunity.
4.2 Oncology
Tα1 enhances tumour antigen presentation and CD8⁺ infiltration; early clinical programmes suggest additive efficacywith PD-1/PD-L1 inhibitors and TACE in HCC, with acceptable tolerability.
4.3 Sepsis/Critical Care
Signals for reversal of immunoparesis (↑ mHLA-DR, ↑ lymphocyte counts) and lower secondary infections in phenotyped (lymphopenic) patients; heterogeneous results emphasize patient selection.
4.4 Vaccinology & Ageing
Consistent adjuvant effects in hypo-responders (older adults, CKD, cirrhosis), including higher seroprotection ratesand more durable cellular immunity.
Evidence quality note: Contemporary, well-controlled multicentre trials exist in hepatology and oncology; critical-care and NAFLD data are emerging and heterogeneous.
5. Emerging Clinical Interests
| Field | Rationale | Current status |
|---|---|---|
| Checkpoint-inhibitor adjunct | Bolster priming, reduce immune exhaustion | Phase 2 signals; larger RCTs ongoing |
| Phenotype-guided sepsis care | Treat lymphopenic immunoparesis | Precision-trial designs in progress |
| NAFLD/NASH | Immune-metabolic re-tuning of liver | Pilot RCTs planned |
| Transplant infectious risk | Improve antiviral defence without rejection | Early feasibility |
| Elderly vaccine response | Raise seroconversion/durability | Positive small trials; policy studies needed |
6. Safety and Tolerability
-
Common: Mild injection-site erythema, transient fatigue, headache, nausea.
-
Immune effects: Generally immunorestorative, not broadly immunosuppressive; monitor in autoimmuneconditions (rare flares).
-
Hepatic: Typically improves rather than worsens transaminases in hepatitis cohorts.
-
Drug interactions: Additive with interferons and antivirals; no known CYP issues.
-
Special populations: Use caution in transplant (balance infection risk vs rejection), pregnancy, and pediatricsettings outside trials.
Comparative safety matrix
| Concern | Thymosin α1 (synthetic) | Thymalin (bovine extract) | Interferon-α (for HBV/HCV, historical) |
|---|---|---|---|
| Composition | Single 28-aa peptide | Multi-peptide mixture | Cytokine biologic |
| Immunologic action | Host-directed restoration | Broad immunocorrection | Potent antiviral/immunostimulant |
| AE profile | Mild, injection-site/fatigue | Variable; extract-related | Flu-like syndrome, depression, cytopenias |
| Regulatory footprint | Approved ex-US/EU | Regional (RU/CIS) | Global (historical hepatitis use) |
7. Regulatory Landscape
-
Approved (ex-US/EU): Adjunct treatment for chronic HBV/HCV, immune restoration, and vaccine adjuvancyin multiple countries (e.g., parts of Asia, ME/LatAm).
-
United States/EU: Not FDA/EMA-approved; accessible only via clinical trials or special pathways.
-
Quality: Use GMP-certified thymalfasin; avoid unregulated “research peptide” sources.
8. Future Directions
-
Phenotype-driven trials in lymphopenic sepsis, checkpoint-inhibitor combinations, and NAFLD/NASH with immune and imaging endpoints.
-
Biomarker-guided therapy (e.g., mHLA-DR, lymphocyte counts, cytokine signatures) to select responders and time courses.
-
Combination immunotherapy frameworks (with PD-1/PD-L1, oncolytic viruses, TLR agonists) to deepen antitumour immunity.
-
Public-health integration as adjuvant for hard-to-immunize groups (elderly, CKD, cirrhosis, immunosuppressed).
-
Mechanistic mapping of TLR bias and downstream IRF vs NF-κB balance to predict efficacy and safety.
Selected References
-
Hepatology; Journal of Hepatology — Thymosin α1 in chronic viral hepatitis (monotherapy and combination).
-
Vaccine; Human Vaccines & Immunotherapeutics — Adjuvant effects on seroconversion and T-cell responses.
-
Cancer Immunology Research; Annals of Oncology — Combinatorial immunotherapy data (checkpoint inhibitors, HCC regimens).
-
Critical Care; Intensive Care Medicine — Reversal of immunoparesis in sepsis and critical illness.
-
Gerontology; Mechanisms of Ageing and Development — Immunosenescence trials and infection outcomes.
-
Bone Marrow Transplantation; Supportive Care in Cancer — Onco-hematology supportive-care studies.
-
Hepatology Communications; Liver International — Early NAFLD/NASH investigations and biomarkers.