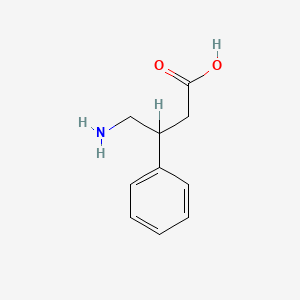
Phenibut HCL 100x200mg
Pickup currently not available
NOT FOR HUMAN CONSUMPTION
Phenibut (β-phenyl-γ-aminobutyric acid) is a synthetic derivative of the neurotransmitter gamma-aminobutyric acid (GABA). Developed in the Soviet Union in the 1960s, phenibut was originally utilized clinically to treat anxiety, insomnia, stress, and other psychiatric or neurological conditions. Phenibut hydrochloride (Phenibut HCl) is the most commonly available formulation, notable for its improved water solubility and bioavailability compared to the free acid form.
Chemical and Pharmacological Profile
-
Chemical Name: β-phenyl-γ-aminobutyric acid hydrochloride
-
Molecular Formula: C₁₀H₁₃NO₂·HCl
-
Classification: GABA analog; central nervous system depressant/anxiolytic
-
Administration: Oral, typically as powder, capsule, or tablet.
Mechanism of Action
Phenibut primarily exerts its effects by modulating GABAergic and dopaminergic systems:
1. GABA-B Receptor Agonism
-
Phenibut acts as a selective agonist at the GABA-B receptor.
-
Activation of GABA-B receptors produces anxiolytic (anti-anxiety), sedative, and muscle-relaxing effects.
2. Indirect Effects on Dopamine Levels
-
Phenibut has mild stimulatory effects on dopamine transmission.
-
Elevated dopamine levels in select brain regions may enhance motivation, cognition, mood, and sociability.
3. Mild Effects on GABA-A Receptors
-
Phenibut may have minimal action at GABA-A receptors, primarily at higher dosages, potentially contributing to sedation.
Clinical and Therapeutic Applications
Historically and clinically (particularly in Eastern Europe and Russia), phenibut is used for various therapeutic purposes:
1. Anxiety Reduction (Anxiolytic)
-
Utilized clinically to relieve symptoms of social anxiety, generalized anxiety, panic disorders, and stress-related anxiety conditions.
2. Improvement of Sleep Quality (Hypnotic/Sedative Effects)
-
Prescribed to treat insomnia, promote deep sleep, and regulate sleep patterns.
3. Cognitive and Mood Enhancements
-
Anecdotally used for cognitive enhancement (nootropic), improvement of social confidence, enhanced mood stability, and stress resilience.
4. Other Potential Clinical Uses
-
Reportedly helpful in managing withdrawal symptoms from alcohol or benzodiazepines (in supervised medical contexts).
-
May alleviate symptoms of post-traumatic stress disorder (PTSD), though clinical studies remain limited.
Dosage and Administration Guidelines (Non-medical & Anecdotal)
Typical dosages used clinically and experimentally (for reference only; individual response varies significantly):
-
Low dose: 250–500 mg/day (for mild anxiety, stress relief, subtle mood improvement)
-
Moderate dose: 500–1500 mg/day (for more pronounced anxiolytic effects, social anxiety relief, sleep support)
-
High dose: 1500–2500 mg/day (heavy sedation, stronger anxiolytic effects)
Important Usage Notes:
-
Phenibut has a delayed onset of action, typically 1–3 hours, and a prolonged duration, usually lasting 8–12 hours.
-
Due to tolerance and dependence risks, phenibut use is commonly limited to 1–3 times per week maximum.
Safety Profile and Side Effects
Phenibut presents potential risks and side effects, especially with chronic or high-dose use:
Common Acute Side Effects (Low-to-Moderate Doses):
-
Sedation or excessive drowsiness
-
Dizziness or impaired coordination
-
Nausea or gastrointestinal discomfort
-
Mild headaches
High-Dose Adverse Effects:
-
Pronounced sedation or cognitive impairment
-
Severe dizziness or nausea
-
Balance issues, ataxia, impaired motor function
-
Agitation, irritability, anxiety rebound upon withdrawal
Tolerance, Dependence, and Withdrawal Risks:
-
Chronic or frequent use rapidly leads to tolerance, requiring escalating doses for similar effects.
-
Physical dependence can develop quickly; abrupt cessation after chronic use can trigger severe withdrawal symptoms, including rebound anxiety, insomnia, agitation, tremors, confusion, tachycardia, and in severe cases, psychosis or seizures.
-
Phenibut withdrawal can be severe and may require medical supervision.
Contraindications and Drug Interactions
Contraindications:
-
History of substance abuse or dependence
-
Liver or kidney impairment (due to potential metabolic strain)
-
Pregnancy or lactation (safety data lacking)
-
Psychiatric disorders (especially bipolar disorder or schizophrenia), unless under medical supervision
Dangerous Interactions:
-
Alcohol and other central nervous system depressants (benzodiazepines, opioids, barbiturates) significantly enhance sedation and respiratory depression risk.
-
Stimulants or dopamine-enhancing drugs might lead to unpredictable psychological effects or overstimulation.
Legal and Regulatory Status
-
Russia and Eastern Europe: Phenibut is approved as a prescription medication (brands: Noofen, Fenibut, Anvifen).
-
United States, Canada, Australia, Europe: Sold as an unregulated dietary supplement or "research chemical." Not approved or regulated by FDA or analogous regulatory agencies; availability varies by region, legality is evolving.
-
Certain countries have begun regulating or banning phenibut due to health concerns, including Australia and parts of the EU.
Current Research Status
-
Limited robust clinical trials exist, especially in Western scientific literature.
-
Most evidence supporting therapeutic benefits is derived from Russian-language literature and anecdotal reports.
-
More controlled trials are required to validate therapeutic claims, determine optimal dosages, and establish long-term safety and efficacy.
Practical Recommendations and Harm Reduction
To minimize risks associated with Phenibut:
-
Use infrequently (ideally not exceeding 1–2 times weekly).
-
Limit dosage (start with the lowest effective dose).
-
Avoid chronic use due to rapid development of tolerance and dependence.
-
Never abruptly discontinue chronic phenibut use; taper slowly under medical guidance.
-
Avoid combining phenibut with alcohol, benzodiazepines, opioids, or other CNS depressants.
Summary of Benefits and Risks
| Benefits (Potential) | Risks (Significant) |
|---|---|
| Anxiety and stress relief | High risk of dependence & withdrawal |
| Sleep improvement | Rapid tolerance development |
| Social anxiety reduction | Risk of severe withdrawal symptoms |
| Mood enhancement | Potential adverse interactions (alcohol, CNS depressants) |
| Cognitive or motivational boost (anecdotal) | Insufficient research on long-term safety |
References
-
Lapin, I. (2001). "Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug." CNS Drug Reviews, 7(4), 471-481.
-
Samokhvalov, A. V., et al. (2013). "Phenibut dependence." BMJ Case Reports, 2013:bcr2012008381.
-
Owen, D. R., et al. (2016). "Phenibut dependence and withdrawal: an emerging problem." Journal of Clinical Psychopharmacology, 36(6), 686-688.
-
Shulgina, G. I. (1986). "On neurotransmitter mechanisms of reinforcing effect of phenibut." Eksperimental'naia i Klinicheskaia Farmakologiia, 49(5), 7-11.