PEG-MGF 5mg vial
Pickup currently not available
NOT FOR HUMAN CONSUMPTION
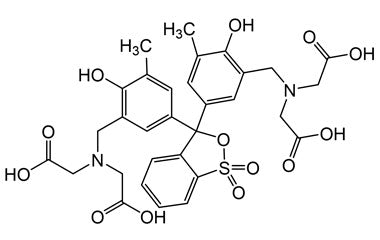
PEG-MGF stands for Pegylated Mechano Growth Factor, a modified form of MGF (a splice variant of IGF-1, Insulin-like Growth Factor 1). MGF is naturally produced in muscle tissue in response to physical exercise or muscle damage. The pegylation of MGF involves attaching polyethylene glycol (PEG) to the molecule, greatly extending its half-life and stability in circulation, allowing for less frequent dosing and prolonged biological activity.
Chemical and Biological Properties
-
Full Name: Pegylated Mechano Growth Factor
-
Structure: PEG-conjugated synthetic peptide, derived from IGF-1 (Ec domain, exon 5 splice variant).
-
Molecular Modification: PEGylation significantly increases peptide stability, reduces proteolytic degradation, and prolongs the half-life from mere minutes (native MGF) to hours or days.
-
Administration Route: Primarily administered through subcutaneous injection, sometimes intramuscularly.
Mechanism of Action
PEG-MGF exerts anabolic and regenerative effects primarily through two key mechanisms:
1. Muscle Hypertrophy and Repair
-
MGF activates muscle satellite cells (stem cells within muscle tissue), stimulating proliferation and differentiation.
-
Enhances muscle regeneration, repair, and hypertrophy (growth), particularly after injury or mechanical loading.
2. Anti-apoptotic and Protective Effects
-
May exert protective effects on muscle cells by reducing apoptosis (cell death), contributing to improved muscle preservation during stress or aging.
Unlike systemic IGF-1, PEG-MGF's actions are more targeted, focusing on muscle-specific recovery and regeneration rather than broader metabolic effects.
Potential Therapeutic and Experimental Applications
1. Muscle Recovery and Injury Rehabilitation
-
Promotes faster recovery from muscle injuries, trauma, or extensive physical exertion.
-
Potential clinical use in treating muscle-wasting conditions, sarcopenia, or muscular dystrophy.
2. Enhanced Muscle Growth (Bodybuilding and Athletics)
-
Experimentally used by athletes and bodybuilders to accelerate muscle growth, enhance recovery, and improve physical performance.
-
Typically combined with structured resistance training for optimal outcomes.
3. Anti-Aging and Longevity (Experimental)
-
Investigational interest in combating age-related muscle atrophy and improving muscle function in elderly populations.
Dosing and Administration (Experimental Use)
No standardized medical dosage guidelines exist; however, common experimental practices reported by users include:
-
Dosage Range (Common): 200–400 mcg per injection.
-
Administration Schedule: Typically administered subcutaneously or intramuscularly, once or twice weekly due to the long half-life provided by pegylation.
-
Cycle Length: Usually employed experimentally for 4–6 weeks, followed by breaks to evaluate results and avoid receptor downregulation or tolerance.
Note: These dosing guidelines are anecdotal, derived from non-medical experimental usage; robust clinical evidence remains lacking.
Safety Profile and Side Effects
PEG-MGF appears generally well-tolerated based on limited anecdotal reports; however, due to scarce clinical studies, full safety profiles remain uncertain:
Reported Side Effects (Mild and Rarely Severe):
-
Injection-site discomfort, redness, or mild swelling.
-
Potential transient hypoglycemia (rare), as IGF variants influence glucose metabolism (though far less than systemic IGF-1).
-
Rare reports of water retention or mild fatigue.
Long-term Safety Concerns:
-
Theoretical risks related to IGF pathways (e.g., potential tumor growth stimulation); however, no definitive evidence exists for PEG-MGF specifically.
-
Insufficient long-term clinical data to conclusively assess chronic safety.
Contraindications and Precautions
-
Contraindications: History or current diagnosis of cancer or uncontrolled metabolic disorders (due to theoretical IGF-related risks).
-
Interactions: No well-documented severe drug interactions; caution advised when used alongside insulin or other peptides that alter metabolic processes.
-
Pregnancy/Nursing: Contraindicated due to unknown safety in pregnancy or breastfeeding.
Legal and Regulatory Status
-
PEG-MGF is not approved by regulatory agencies (FDA, EMA, Health Canada) for human therapeutic use.
-
Typically marketed as a "research chemical" or experimental peptide labeled "not for human consumption."
-
Banned by World Anti-Doping Agency (WADA) for competitive athletic use due to performance-enhancing effects.
Current Research Status
Research on PEG-MGF is primarily preclinical or experimental, with limited peer-reviewed human clinical studies available:
-
Preclinical Studies: Animal models confirm significant muscle regenerative potential, hypertrophic effects, and enhanced muscle satellite cell activation.
-
Human Clinical Trials: Very limited; predominantly anecdotal or small-scale observational data available from unregulated use.
-
Research Gaps: Well-designed human studies needed to establish clinical efficacy, safety, optimal dosing protocols, and long-term effects.
Summary of Potential Benefits and Risks
| Potential Benefits | Potential Risks and Limitations |
|---|---|
| Accelerated muscle repair and growth | Limited clinical safety data |
| Enhanced muscle recovery | Theoretical concerns regarding IGF-related pathways |
| Reduced muscle atrophy or degeneration | Uncertain long-term safety profile |
| Targeted muscular regenerative effect | Regulatory uncertainty and restrictions |
Future Directions and Research Needs
-
Clinical trials are required to determine efficacy, dosing guidelines, and safety profiles in humans.
-
Long-term safety studies to assess potential carcinogenic risks or metabolic disturbances.
-
Development of standardized, quality-controlled manufacturing practices and regulatory guidelines to ensure product purity, potency, and safety.
Conclusion
PEG-MGF is a promising synthetic peptide notable for its potential regenerative, anabolic, and anti-catabolic effects, particularly in muscle tissue. While anecdotal and preclinical data suggest significant therapeutic benefits, lack of substantial clinical evidence and safety data currently limits its legitimate therapeutic use. Individuals interested in experimental use must approach PEG-MGF cautiously, understanding the associated regulatory, safety, and efficacy uncertainties.
References
-
Goldspink, G. (2005). "Research on mechano growth factor: its potential for optimising physical training as well as misuse in doping." British Journal of Sports Medicine, 39(11), 787–788.
-
Philippou, A., & Barton, E. R. (2014). "Optimizing IGF-I for skeletal muscle therapeutics." Growth Hormone & IGF Research, 24(5), 157–163.
-
ClinicalTrials.gov database (search "Mechano Growth Factor" or "IGF variants") to monitor potential ongoing or future clinical trials.
Disclaimer:
PEG-MGF is an experimental research compound and is not approved for clinical use by major regulatory bodies. This document is provided for educational purposes and does not constitute medical advice. Always consult a healthcare professional before considering experimental peptides or supplements.