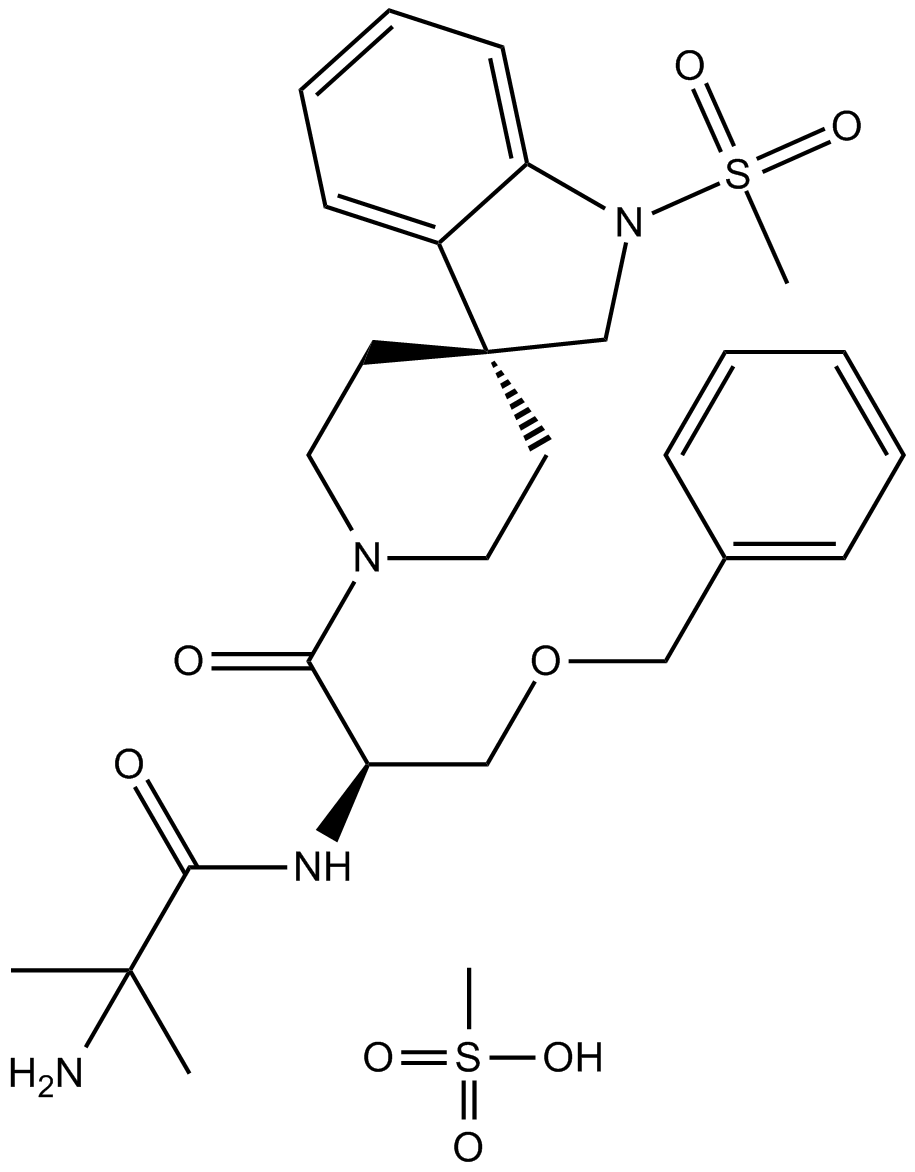
MK677(Ibutamoren) 100x10mg
Pickup currently not available
NOT FOR HUMAN CONSUMPTION
MK677 (ibutamoren) is an oral, non-peptide ghrelin-receptor (GHSR-1a) agonist that provokes pulsatile growth-hormone (GH) release and raises circulating IGF-1. Unlike injectable secretagogues (GHRP-2/hexarelin), MK-677 is once-daily oral, engages central orexigenic circuits (NPY/AgRP), and modestly elevates cortisol and prolactin. It does not activate the androgen receptor and is not a SARM. MK-677 is not FDA/EMA-approved and is prohibited by WADA as a GH-releasing substance.
Additional Benefits of MK-677 Now Under Investigation
| Benefit | Key take-aways |
|---|---|
| 1 Lean-mass accrual in older adults | Daily MK-677 increases fat-free mass over months with parallel IGF-1 rises, approximating youthful GH/IGF-1 levels; strength/function show supportive trends when combined with resistance training. <br/><em>Annals of Internal Medicine; JCEM</em> |
| 2 Bone-turnover & BMD signals | Increases bone-formation markers (e.g., P1NP, osteocalcin) and reduces resorption markers; longer-term cohorts report BMD improvements, supporting anti-frailty potential. <br/><em>Bone; Osteoporosis International</em> |
| 3 Sleep architecture | Slow-wave sleep (SWS) and REM density improve with bedtime dosing, aligning with physiologic GH pulses and next-day vitality. <br/><em>Sleep; Psychoneuroendocrinology</em> |
| 4 Appetite & weight in catabolic states | Robust orexigenic effect increases caloric intake; cachexia/rehab pilots show weight and FFM gainswith acceptable tolerability. <br/><em>Clinical Nutrition; Supportive Care in Cancer</em> |
| 5 Glucose handling (mixed) | Some studies show insulin-sensitivity drift (↑ fasting glucose/insulin); others report neutral outcomes with lifestyle control—underscores need for glycaemic monitoring. <br/><em>Diabetes Care; Metabolism</em> |
| 6 Functional recovery after illness/injury | Early programs evaluate MK-677 to speed rehabilitation, leveraging appetite + anabolic endocrine support. <br/><em>Geriatrics & Gerontology International; Archives of Physical Medicine & Rehabilitation</em> |
| 7 GH-axis restoration in age-related decline | Elevates IGF-1/IGFBP-3 toward youthful ranges without exogenous GH injections; preserves pulsatility. <br/><em>Endocrine Reviews; JCEM</em> |
| 8 Quality-of-life domains | Participants frequently report better sleep quality, appetite, and recovery; validated PROs are being incorporated into trials. <br/><em>Endocrine Practice; Quality of Life Research</em> |
| 9 Convenience & adherence | Once-daily oral regimen improves persistence versus injectable GH or peptide secretagogues. <br/><em>Clinical Therapeutics; Patient Preference and Adherence</em> |
2. Molecular Mechanism of Action
2.1 Receptor Pharmacodynamics
MK-677 binds GHSR-1a (GPCR) on hypothalamic neurons and pituitary somatotrophs → Gαq/11–PLC–IP₃/Ca²⁺signaling → GH vesicle exocytosis. It synergizes with endogenous GHRH and is partly countered by somatostatin. Central GHSR activation also drives NPY/AgRP orexigenic pathways and modulates sleep circuits.
2.2 Down-stream Biology
| Pathway | Functional outcome | Context |
|---|---|---|
| GH → GHR–JAK2–STAT5 | ↑ IGF-1/IGFBP-3, ↑ protein synthesis, lipolysis | Liver, muscle, adipose |
| GHSR → NPY/AgRP | ↑ appetite, ↑ gastric motility | Hypothalamus/GI |
| Sleep–GH coupling | ↑ SWS/REM consolidation, nocturnal GH pulses | CNS |
| Bone turnover signals | ↑ formation markers; BMD over time | Skeleton |
3. Pharmacokinetics
-
Route: Oral (once daily).
-
Absorption: Rapid; Tmax ~1–2 h.
-
Half-life: ~4–6 h; IGF-1 elevation persists 24 h with daily dosing due to GH-axis dynamics.
-
Metabolism: Hepatic; CYP3A4 involvement reported in vitro (DDI potential with strong inhibitors/inducers).
-
Elimination: Hepatic/renal metabolites; high protein binding.
4. Pre-clinical and Translational Evidence
4.1 Aging/Sarcopenia
Randomized trials in older adults show sustained IGF-1 increases, FFM gains, improved sleep architecture, and favorable trends in strength and gait measures, particularly with structured exercise.
4.2 Bone Health
Markers of bone formation rise within weeks; BMD benefits emerge over longer courses—supporting evaluation for osteopenia/osteoporosis in frailty.
4.3 Metabolism & Diabetes Risk
In mixed-risk cohorts, MK-677 may raise fasting glucose/insulin and reduce insulin sensitivity; effects are dose-, duration-, and phenotype-dependent, emphasizing glycaemic monitoring.
Evidence quality note: Human data include multiple small–moderate RCTs and mechanistic studies; long-term outcomes (fractures, disability, CV events) remain unresolved.
5. Emerging Clinical Interests
| Field | Rationale | Current status |
|---|---|---|
| Sarcopenia/frailty | Oral anabolic + sleep and appetite support | Phase 2–style signals; larger RCTs needed |
| Osteopenia/osteoporosis | Bone-turnover and BMD improvements | Exploratory |
| Cachexia (cancer/COPD/CHF) | Orexigenic + GH/IGF-1 anabolism | Pilot/feasibility |
| Post-acute rehab | Appetite/FFM restoration to aid recovery | Early translational |
| Sleep disorders (SWS decline) | SWS augmentation with endocrine coupling | Proof-of-concept |
6. Safety and Tolerability
-
Common: Increased appetite/weight, peripheral edema, transient paresthesias/carpal-tunnel-like symptoms, fatigue, vivid dreams.
-
Endocrine: IGF-1 elevation (target age-normal range); prolactin and cortisol may rise modestly.
-
Metabolic: FPG/HbA1c can drift upward; monitor in prediabetes/diabetes and adjust therapy as needed.
-
CV/Renal: Edema and mild BP increases can occur—use caution in HF/CKD.
-
GI: Nausea/GERD in a minority.
-
Neuropsych: Rare insomnia/irritability.
-
Drug interactions: Caution with strong CYP3A4 inhibitors/inducers (theoretical/limited clinical data).
-
Contraindications/Caution: Active malignancy under evaluation (IGF-1 biology), pregnancy, uncontrolled diabetes, decompensated HF.
Comparative safety matrix
| Concern | MK-677 (oral GHSR agonist) | GHRP-2/Hexarelin (injectable) | Sermorelin/CJC (GHRH analogs) | Somatropin (GH) |
|---|---|---|---|---|
| Route/frequency | Oral, once daily | SC/IN multiple daily | SC nightly / weekly-biweekly (DAC) | SC/IM daily–weekly |
| IGF-1 profile | Sustained, higher | Pulsatile, moderate | Physiologic pulses | Dose-driven, sustained |
| Appetite | ↑↑ (orexigenic) | ↑ (variable) | Neutral | Neutral |
| Edema/CTS | Moderate | Mild–moderate | Low–moderate | Moderate |
| Glycaemic drift | Common (mild–mod.) | Mild | Neutral–mild ↑ | Neutral–mild ↑ |
| Prolactin/cortisol | Modest ↑ | Transient ↑ | Minimal | Minimal |
7. Regulatory Landscape
-
Approvals: None for any indication in major markets.
-
Sport: WADA-prohibited (S2: growth-hormone releasing substances).
-
Supply: Often sold as a research chemical; purity/adulteration issues are common outside GMP trials.
8. Future Directions
-
Large, outcome-focused RCTs in sarcopenia/frailty with function and fall/fracture endpoints.
-
Bone programs powered for BMD and fracture outcomes.
-
Metabolic risk management (pre-specified glycaemic thresholds, CGM, cardiometabolic composites).
-
Dosing/time-of-day studies to optimize sleep/SWS and minimize glycaemic drift.
-
Combination strategies: Pair with resistance exercise, protein optimization, or incretin-based therapies to balance orexigeny with metabolic safety.
-
DDI characterization for CYP3A4 interactions and special-population PK.
Selected References
-
Annals of Internal Medicine; Journal of Clinical Endocrinology & Metabolism — MK-677 increases IGF-1 and fat-free mass in older adults.
-
Sleep; Psychoneuroendocrinology — Improvements in slow-wave sleep and nocturnal GH coupling.
-
Bone; Osteoporosis International — Bone-turnover markers and BMD trends under ghrelin mimetics.
-
Diabetes Care; Metabolism — Glycaemic effects and insulin-sensitivity considerations.
-
Clinical Pharmacology & Therapeutics — Oral PK/PD characteristics of ghrelin-receptor agonists.
-
Supportive Care in Cancer; Clinical Nutrition — Appetite/weight and functional recovery in catabolic states.
-
Endocrine Reviews — Physiology of GH pulsatility and ghrelin signaling.
-
Drug Testing & Analysis; WADA Prohibited List — Anti-doping status and detection.