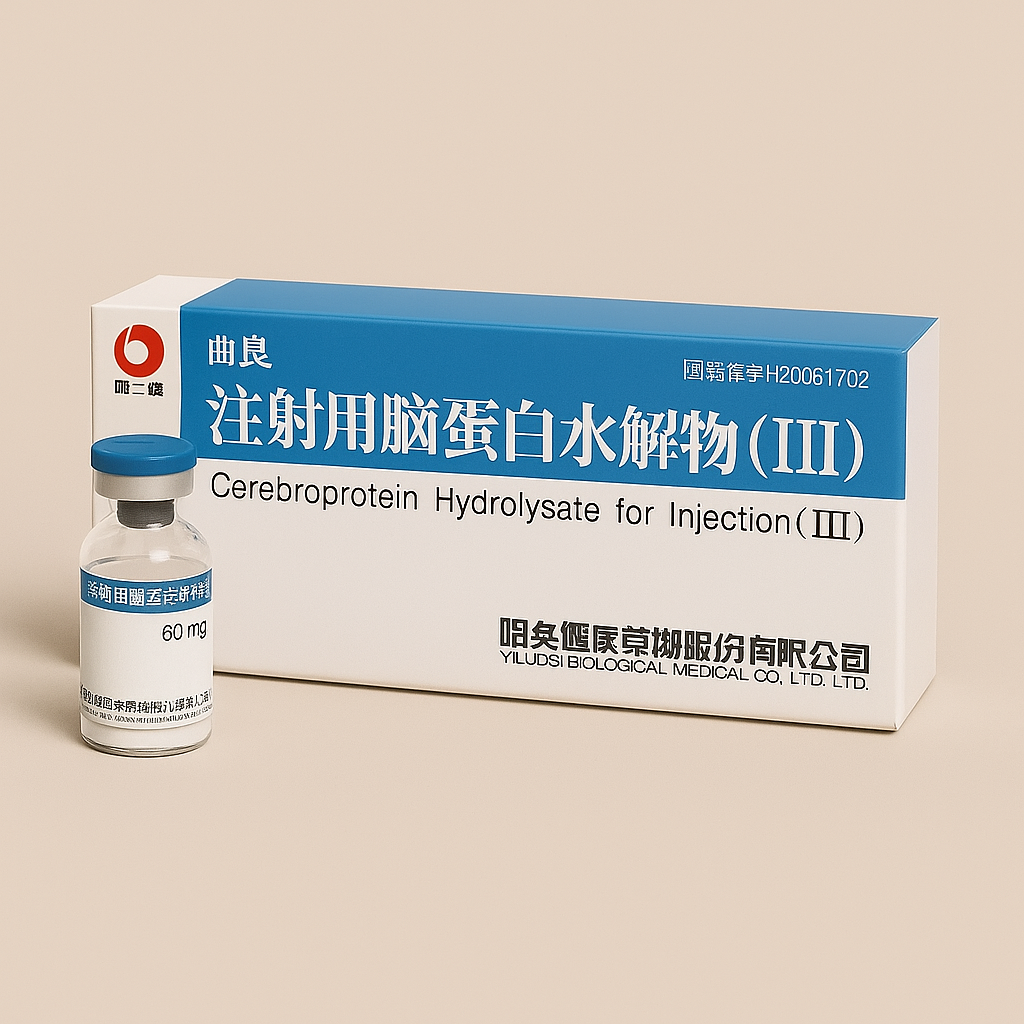
Cerebrolysin 60mg vial
Pickup currently not available
NOT FOR HUMAN CONSUMPTION
Cerebrolysin is a standardized porcine brain–derived peptide and amino-acid preparation (low–molecular-weight neuropeptides <10 kDa) formulated for parenteral use. It exerts neurotrophic-like activity—functionally mimicking BDNF/NGFsignaling—promoting neuronal survival, synaptogenesis, neurogenesis, and plasticity while dampening excitotoxicity, oxidative stress, and neuroinflammation. It is not FDA-approved; it is marketed in several countries (ex-US/UK) for stroke recovery, traumatic brain injury (TBI), and cognitive impairment based on regional evidence and guidelines.
Additional Benefits of Cerebrolysin Now Under Investigation
| Benefit | Key take-aways |
|---|---|
| 1 Post-ischemic stroke functional recovery | Meta-analyses and RCTs report improved global disability and NIHSS with early IV courses (e.g., 30 mL/day × 10–20 days), particularly when paired with structured rehab; heterogeneity and risk of bias remain. <br/><em>Stroke; Journal of Stroke & Cerebrovascular Diseases; European Stroke Journal</em> |
| 2 Traumatic brain injury (moderate–severe) | Signals for better Glasgow Outcome Scale-Extended, faster cognitive/motor gains, and lower mortality trends in some cohorts using 10–50 mL/day cycles; larger contemporary trials are needed. <br/><em>Neurorehabilitation & Neural Repair; Journal of Neurotrauma</em> |
| 3 Vascular/mixed cognitive impairment | Modest attention/executive and memory improvements across small RCTs; effects strengthen when combined with multidomain cognitive rehab. <br/><em>CNS Drugs; International Journal of Geriatric Psychiatry</em> |
| 4 Alzheimer’s disease (symptomatic) | Add-on to standard care shows short-term cognitive and global-function benefits in mild–moderate AD; durability and disease-modification are unproven. <br/><em>Alzheimer’s Research & Therapy; Journal of Alzheimer’s Disease</em> |
| 5 Aphasia & post-stroke language | Pilot trials indicate greater gains on aphasia batteries when Cerebrolysin is given concurrently with intensive speech therapy. <br/><em>Aphasiology; Neuropsychological Rehabilitation</em> |
| 6 Motor plasticity & gait | Enhanced Fugl-Meyer, Barthel Index, gait speed and DTI/fMRI plasticity markers when paired with task-specific physiotherapy. <br/><em>Neurorehabilitation & Neural Repair; Restorative Neurology & Neuroscience</em> |
| 7 Post-ICU/encephalopathy recovery | Observational programs suggest faster cognitive/functional rebound after toxic-metabolic encephalopathy; controlled evidence is preliminary. <br/><em>Critical Care Medicine; Frontiers in Neurology</em> |
| 8 Neuroprotection biomarkers | In models and small human studies: ↑BDNF, ↓NfL, ↓MDA/oxidative stress, and ↓pro-inflammatory cytokines, paralleling clinical improvements. <br/><em>Molecular Neurobiology; Frontiers in Aging Neuroscience</em> |
| 9 Combination neurorehab strategies | Additive effects with robot-assisted therapy, NMES, rTMS/tDCS, and early mobilization, supporting a “drug-plus-dose-dense-rehab” paradigm. <br/><em>Clinical Neurophysiology; Archives of Physical Medicine & Rehabilitation</em> |
2. Molecular Mechanism of Action
2.1 Pharmacodynamics
Cerebrolysin’s peptide fractions activate Trk receptors (TrkB/TrkA-like) and downstream PI3K–Akt and MAPK–ERK cascades, enhancing CREB signaling → synaptic protein expression (synapsin, PSD-95), neurite outgrowth, and anti-apoptosis (Bcl-2↑/caspase↓). Additional actions include NMDA/GABA balance (anti-excitotoxic), microglial modulation (NF-κB down-tuning), mitochondrial support, and amyloid/tau pathway normalization in models.
2.2 Down-stream Biology
| Pathway | Functional outcome | Context |
|---|---|---|
| BDNF/TrkB → PI3K–Akt–CREB | Neuronal survival, LTP, synaptogenesis | Hippocampus/cortex |
| MAPK–ERK | Plasticity, learning, neurogenesis | Neurorehab windows |
| NMDA/GABA re-balancing | Anti-excitotoxicity, network stability | Acute/subacute injury |
| NF-κB / NLRP3 restraint | ↓ Microglial cytokines, ↓ inflammation | Stroke/TBI, AD |
| Oxidative stress control | ↓ ROS/lipid peroxidation; mitochondrial support | Ischemia–reperfusion |
| Aβ/tau modulation | ↓ Amyloid toxicity, ↓ tau hyper-phosphorylation (models) | AD-like settings |
3. Pharmacokinetics
-
Composition: Low-MW neuropeptides and free amino acids; parenteral only.
-
BBB access: Peptides <10 kDa and fragments cross the BBB; pharmacodynamic effects outlast plasma exposure.
-
Half-life: Short (minutes–hours) for peptide components; signaling changes persist for days–weeks.
-
Dosing in studies: IV infusion 5–50 mL/day (often 30 mL/day) for 10–20 days, repeated every 1–3 months in subacute/chronic phases; IM 5 mL used in some protocols.
4. Pre-clinical and Translational Evidence
4.1 Stroke
Rodent and primate stroke models show smaller infarcts, better neurologic scores, and enhanced peri-infarct plasticity. Human RCTs/meta-analyses suggest functional benefits with early use + rehab, but trial heterogeneity(timing, dose, endpoints) tempers certainty.
4.2 Traumatic Brain Injury
Improved consciousness metrics, cognition, and activities of daily living reported in several trials/series; multi-center, modern-design RCTs are warranted.
4.3 Cognitive Disorders
In vascular/mixed impairment and mild–moderate AD, repeated courses yield modest cognitive and global-function gains; disease-modifying effects remain unproven.
Evidence quality note: The totality of evidence is supportive but heterogeneous (regional RCTs, varying quality). Benefits appear greatest when combined with early, intensive rehabilitation and standardized dosing.
5. Emerging Clinical Interests
| Field | Rationale | Current status |
|---|---|---|
| Aphasia/upper-limb recovery | Trophic plasticity + task-specific training | Phase 2–style signals |
| Post-ICU cognitive dysfunction | Anti-inflammatory/mitochondrial support | Observational/pilot |
| Long-COVID cognitive issues | Neuroinflammatory modulation | Exploratory |
| Perioperative neurocognitive disorder | Synaptic resilience | Concept/feasibility |
| Parkinson’s-related cognitive decline | Synaptic/mitochondrial support | Early studies |
6. Safety and Tolerability
-
Common: Injection-site reactions, transient agitation/anxiety, dizziness, headache, flushing, sweating, insomnia; generally mild.
-
Less common/rare: Allergic reactions, blood-pressure lability; avoid in known hypersensitivity.
-
Seizure risk: Use caution in uncontrolled epilepsy (excitability symptoms possible).
-
Drug interactions: No major CYP interactions expected; monitor with CNS-active regimens (sedatives/stimulants) and antihypertensives.
-
Geriatrics/polymedication: Good short-course tolerability in studies; continue routine BP and neurostatuschecks.
Comparative safety matrix
| Concern | Cerebrolysin | Citicoline (CDP-choline) | Edaravone |
|---|---|---|---|
| Route | IV/IM cycles | Oral/IV | IV |
| Evidence focus | Stroke/TBI, VCI/AD (symptomatic) | Stroke recovery, cognition | ALS (JP), stroke (select regions) |
| AEs | Mild CNS/vasomotor | GI, headache (mild) | Infusion reactions, renal risk |
| Role | Neurotrophic + rehab adjunct | Membrane/choline donor | Free-radical scavenger |
7. Regulatory Landscape
-
Status: Not FDA/EMA-approved; marketed in several countries as an injectable neuropeptide preparation for stroke/TBI/cognitive impairment.
-
Guidance: Best supported as an adjunct to structured neurorehabilitation with early initiation and repeat cycles where locally permitted.
8. Future Directions
-
Modern, adequately powered RCTs with standardized dosing (e.g., 30 mL/day × 10–20 days) and core outcome sets (mRS/NIHSS, Fugl-Meyer, language batteries, PROMs).
-
Precision timing: Initiation within days of stroke/TBI; evaluate dose–response and cycle frequency.
-
Biomarkers & imaging: Serum/CSF BDNF, NfL, inflammatory panels; DTI/fMRI for network plasticity; digital mobility/cognition endpoints.
-
Combination therapy: Pair with high-intensity rehab, speech therapy, robotics, rTMS/tDCS; examine synergyand sequencing.
-
Real-world registries: Safety, adherence, and comparative effectiveness vs citicoline, memantine, donepezil, and standard rehab.
Selected References
-
Stroke; European Stroke Journal; Journal of Stroke & Cerebrovascular Diseases — Acute/subacute stroke trials and meta-analyses of Cerebrolysin.
-
Neurorehabilitation & Neural Repair; Archives of Physical Medicine & Rehabilitation — Functional recovery, motor outcomes, and rehab synergy.
-
Journal of Neurotrauma; Brain Injury — TBI cohorts and functional/cognitive endpoints.
-
CNS Drugs; International Journal of Geriatric Psychiatry — Vascular/mixed cognitive impairment and safety in older adults.
-
Alzheimer’s Research & Therapy; Journal of Alzheimer’s Disease — Add-on symptomatic effects in mild–moderate AD.
-
Molecular Neurobiology; Frontiers in Aging Neuroscience — Mechanistic work: neurotrophic signaling, inflammation, oxidative stress, biomarkers.
-
Restorative Neurology & Neuroscience; Clinical Neurophysiology — Plasticity measures (DTI, fMRI) and combination neuromodulation.