ACP-105 100x10mg
Pickup currently not available
NOT FOR HUMAN CONSUMPTION
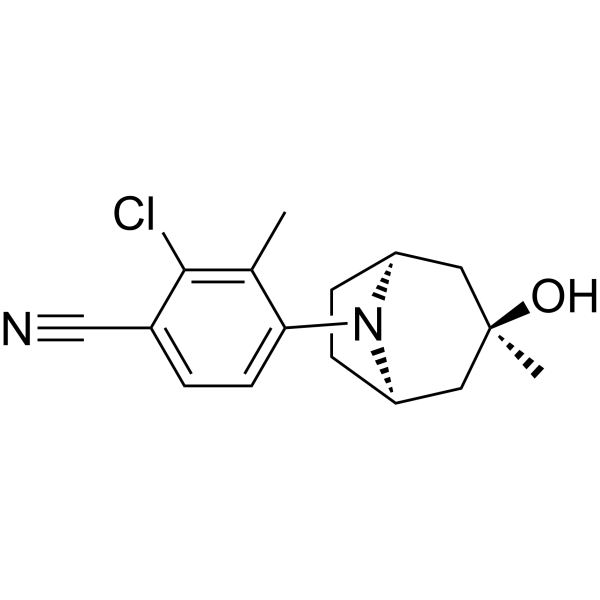
ACP-105 is a novel, selective androgen receptor modulator (SARM) initially developed by Acadia Pharmaceuticals. It has gained attention primarily due to its potential anabolic effects on muscle and bone tissues, combined with minimal androgenic side effects typically associated with traditional anabolic steroids. ACP-105 remains experimental, with promising preclinical results indicating therapeutic potential for muscle wasting, osteoporosis, and certain endocrine conditions.
Chemical and Biological Properties
-
Chemical Name: ACP-105
-
Class: Nonsteroidal Selective Androgen Receptor Modulator (SARM)
-
Administration Route: Primarily oral, providing convenient systemic administration with potential selectivity for muscle and bone tissue.
Mechanism of Action
ACP-105 functions through selective modulation of androgen receptors (AR):
1. Tissue-Selective Androgenic Activity
-
Potent agonist of AR, exhibiting anabolic activity selectively in muscle and bone tissues.
-
Minimal activation of AR in tissues such as the prostate and seminal vesicles, thus reducing common androgenic side effects.
2. Anabolic and Anti-Catabolic Effects
-
Promotes muscle growth and hypertrophy by enhancing protein synthesis and reducing protein degradation.
-
Protects muscle and bone tissue from wasting and degradation, particularly beneficial in cachexia, osteoporosis, and sarcopenia.
3. Improved Bone Density
-
Increases bone mineral density, potentially useful in treating osteoporosis or fracture recovery.
Potential Therapeutic and Experimental Applications
ACP-105 has primarily been researched experimentally in the following contexts:
1. Muscle Wasting and Sarcopenia
-
Shown significant anabolic potential in preventing muscle atrophy and promoting muscle growth in preclinical studies.
-
Potential therapeutic candidate for muscle-wasting conditions such as cachexia (associated with chronic diseases or aging).
2. Osteoporosis and Bone Health
-
Demonstrated promising results in increasing bone mineral density and strength in preclinical rodent models.
-
Potentially valuable as an alternative or adjunctive treatment for osteoporosis or recovery from fractures.
3. Hormone Replacement and Hypogonadism
-
Experimental interest in ACP-105 as a potential alternative to traditional testosterone replacement therapy (TRT), providing anabolic benefits with fewer androgenic risks.
4. Athletic Performance Enhancement (Experimental)
-
Anecdotally explored in performance enhancement contexts to promote lean muscle mass, improve strength, and enhance recovery with fewer side effects than traditional steroids.
Dosage and Administration (Experimental Context)
ACP-105 is not approved by any regulatory agency; thus, standardized human dosing guidelines do not exist. Based on limited anecdotal experimental use, typical dosages are:
-
Experimental Oral Dosage: 5–20 mg/day, most commonly around 10 mg/day.
-
Administration Frequency: Usually taken once daily due to oral bioavailability and pharmacokinetics.
-
Experimental Cycle Duration: Typically administered experimentally for 4–8 weeks, followed by breaks or discontinuation periods.
Note: These dosages reflect anecdotal experimental practices; rigorous clinical dosing data remain unavailable.
Safety Profile and Side Effects
Due to the limited number of human studies, safety data for ACP-105 remain largely preclinical:
Commonly Reported Side Effects (Rare and Mild Anecdotal Reports):
-
Mild headaches or transient fatigue.
-
Minor suppression of natural testosterone production (common with SARMs, but reportedly less severe compared to anabolic steroids).
-
Mild gastrointestinal discomfort (rare).
Potential Long-term Safety Concerns:
-
Limited data regarding long-term effects, particularly relating to endocrine disruption, cardiovascular impacts, or liver function.
-
No data available on potential carcinogenic risks or reproductive toxicity in humans.
Contraindications and Precautions
-
Contraindications:
-
Pregnancy and breastfeeding due to unknown safety.
-
History of hormone-sensitive cancers (e.g., prostate or breast cancer).
-
-
Precautions:
-
Potential suppression of endogenous testosterone; monitoring hormone levels recommended.
-
Individuals with pre-existing liver, renal, or cardiovascular conditions should exercise caution.
-
Legal and Regulatory Status
-
Regulatory Approval: ACP-105 is not approved by regulatory authorities (FDA, EMA, Health Canada) for human therapeutic use.
-
Currently classified and sold exclusively as a "research chemical," labeled "not for human consumption."
-
Explicitly banned by World Anti-Doping Agency (WADA) due to potential performance-enhancing effects.
Current Research Status and Evidence
-
Preclinical Research:
-
Animal studies indicate potent anabolic and bone-protective properties with favorable tissue-selective androgenic profiles.
-
-
Human Clinical Trials:
-
No large-scale human clinical trials available; research primarily preclinical and exploratory.
-
Clinical data are urgently needed to establish definitive efficacy, safety, dosing guidelines, and clinical potential.
-
-
Limitations and Research Needs:
-
Lack of human safety and efficacy data from well-controlled clinical trials.
-
Need for long-term studies to evaluate chronic safety, endocrine effects, and systemic impacts.
-
Summary of Potential Benefits and Risks
| Potential Benefits | Potential Risks and Limitations |
|---|---|
| Muscle growth and strength enhancement | Lack of human clinical safety data |
| Prevention of muscle wasting and sarcopenia | Potential suppression of natural testosterone |
| Improved bone density and fracture recovery | Unclear long-term endocrine and systemic safety risks |
| Selective anabolic activity with fewer androgenic side effects | Regulatory uncertainty and legal restrictions |
Future Directions and Research Needs
-
Rigorous human clinical trials to confirm therapeutic efficacy, determine optimal dosing, and assess safety.
-
Long-term safety evaluations focused on endocrine, cardiovascular, hepatic, and carcinogenic risks.
-
Exploration of ACP-105’s clinical potential in muscle-wasting diseases, osteoporosis, hormone replacement therapy, and related endocrine conditions.
Conclusion
ACP-105 is a promising experimental SARM exhibiting potent anabolic activity in muscle and bone tissues with minimal androgenic side effects in preclinical studies. Despite the therapeutic potential indicated by preliminary animal data and anecdotal human reports, significant gaps remain, including lack of robust clinical trials, uncertainty regarding long-term safety, and regulatory restrictions. Individuals interested in experimental use of ACP-105 should proceed cautiously, recognizing its current status as an unapproved research compound with limited human safety data.
References
-
Vajda, E. G., et al. (2009). "Pharmacokinetics and pharmacodynamics of ACP-105, a novel nonsteroidal selective androgen receptor modulator." Journal of Clinical Pharmacology, 49(10), 1196–1206.
-
Gao, W., et al. (2005). "Preclinical profile of ACP-105, a novel selective androgen receptor modulator (SARM)." Endocrinology, 146(9), 4235–4244.
-
Solomon, Z. J., & Mirkin, S. (2008). "Selective androgen receptor modulators: Current knowledge and clinical applications." Molecular and Cellular Endocrinology, 289(1-2), 42–48.
Disclaimer:
This comprehensive review is provided exclusively for educational purposes. ACP-105 is an experimental research compound and is not approved by health regulatory authorities for therapeutic human use. Always consult qualified healthcare providers before considering experimental compounds or supplements.