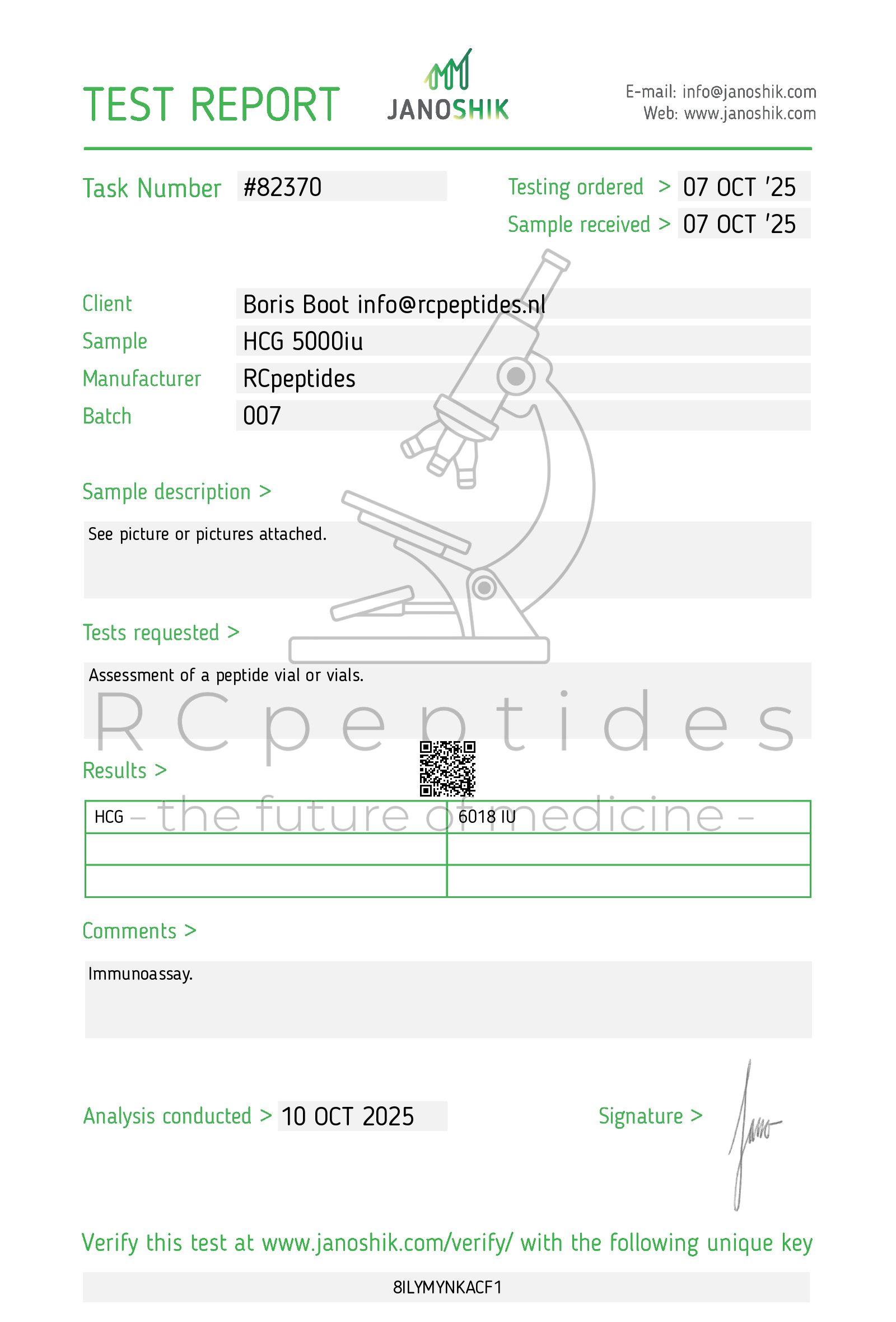
Human chorionic gonadotropin(HCG) 5000iu vial
Afhalen is op dit moment niet beschikbaar
NOT FOR HUMAN CONSUMPTION
Human chorionic gonadotropin(HCG) a glycoprotein hormone (α/β subunits) that acts as a potent LH-receptor (LHCGR) agonist. In ovaries it triggers final oocyte maturation/ovulation and supports the corpus luteum; in testes it stimulates Leydig cells → testosterone and, with FSH, spermatogenesis. hCG is prescription-only; formulations include urinary-derived (u-hCG) and recombinant (r-hCG).
Additional Benefits of hCG Now Under Investigation
| Benefit | Key take-aways |
|---|---|
| 1 Male hypogonadotropic hypogonadism (HH) | hCG restores intratesticular T and supports spermatogenesis when combined with FSH; monotherapy can normalize serum T and libido/energy in HH while preserving fertility potential. <br/><em>JCEM; Endocrine Reviews</em> |
| 2 Fertility preservation during testosterone therapy | Low-dose adjunct hCG can maintain intratesticular T, testicular volume, and sperm production in men receiving exogenous testosterone who wish to avoid azoospermia. <br/><em>Fertility and Sterility; Andrology</em> |
| 3 Induction of spermatogenesis after AAS suppression | Post–anabolic steroid or prolonged TRT, hCG ± FSH protocols help re-initiate spermatogenesis over months. <br/><em>Human Reproduction; Translational Andrology & Urology</em> |
| 4 Prepubertal cryptorchidism (select cases) | hCG can promote testicular descent in some boys (variable success); surgical orchiopexy remains standard. <br/><em>Pediatrics; European Urology</em> |
| 5 Female ART trigger | In IVF, r-hCG/u-hCG reliably triggers final oocyte maturation; kisspeptin or GnRH agonistmay be used in high-OHSS-risk patients. <br/><em>Human Reproduction Update; Reproductive Biomedicine Online</em> |
| 6 Luteal support (adjunct) | hCG can support early luteal progesterone production; modern programs often prefer progesterone to reduce OHSS risk. <br/><em>F&S Reviews; Cochrane</em> |
| 7 Hypogonadism with fertility intent | In functional/secondary hypogonadism (obesity, opioids), hCG can raise T while retaining fertility, sometimes avoiding TRT. <br/><em>Frontiers in Endocrinology; Clinical Endocrinology</em> |
| 8 Diagnostic uses | hCG stimulation tests assess Leydig cell reserve or steroidogenesis in ambiguous genitalia/CAH workups. <br/><em>Best Practice & Research Clinical Endocrinology & Metabolism</em> |
| 9 Onco-fertility | In men treated for pituitary/cranial tumors, hCG±FSH helps re-establish fertility when gonadotropins recover poorly. <br/><em>Annals of Oncology; Endocrine Connections</em> |
2. Molecular Mechanism of Action
2.1 Receptor Pharmacodynamics
-
LHCGR (GPCR) agonist → Gs → cAMP/PKA → steroidogenesis (↑ StAR, CYP11A1) and gamete maturation.
-
In testes: Leydig cells → testosterone; Sertoli support via paracrine crosstalk (FSH still required for full spermatogenesis).
-
In ovaries: The “LH surge mimic” for oocyte maturation, luteinization, and progesterone output.
2.2 Down-stream Biology
| Pathway | Functional outcome | Context |
|---|---|---|
| cAMP/PKA → StAR/CYP enzymes | ↑ Testosterone / ↑ Progesterone | Testis / Ovary |
| Paracrine Sertoli support | Spermatogenic progression (with FSH) | Testis |
| Cumulus expansion/meiotic resumption | Final oocyte maturation | ART trigger |
3. Pharmacokinetics
-
Route: SC or IM.
-
Half-life: u-hCG ~24–36 h; r-hCG similar, enabling once-every-1–3-day dosing (men) or single trigger dose (women).
-
Detection: Prolonged biologic activity; relevant for anti-doping (detectable for days).
4. Pre-clinical and Clinical Evidence
4.1 Male HH & Fertility
Sequential hCG → +FSH regimens restore T, testicular volume, and sperm counts; pregnancy rates improve over 6–18 months depending on baseline testicular size, cryptorchidism history, and adherence.
4.2 Men on TRT desiring fertility
Adjunct low-dose hCG maintains intratesticular T and mitigates testicular atrophy; if azoospermic, add FSH and time.
4.3 Female ART
r-hCG is a gold-standard trigger; however, OHSS risk rises in high responders—GnRH-agonist trigger or kisspeptinmay be safer in such cases.
Evidence quality note: Strong, guideline-level support for HH, ART trigger, and fertility preservation; variable/limited utility for cryptorchidism and functional hypogonadism depends on phenotype.
5. Emerging Clinical Interests
| Field | Rationale | Status |
|---|---|---|
| Opioid-induced androgen deficiency | Preserve fertility, raise T without TRT | Small studies |
| Obesity-related secondary hypogonadism | LHCGR drive with lifestyle/GLP-1 adjuncts | Exploratory |
| Female hypoactive sexual desire (hCG-progesterone milieu) | Hormonal orchestration hypotheses | Very early |
| hCG micro-dosing in ART | Fine-tuning luteal support | Program-dependent |
6. Safety and Tolerability
-
Common: Injection-site pain, headache, mood lability, fatigue, acne/oiliness (from ↑ androgens in men), breast tenderness.
-
Men: Gynecomastia (via aromatization), erythrocytosis (rare vs TRT), worsening OSA in predisposed, testicular ache early in therapy.
-
Women: Ovarian hyperstimulation syndrome (OHSS) risk post-trigger (ascites, hemoconcentration), multiple gestation risk if not carefully managed.
-
Cardio-metabolic: Monitor BP, lipids, glucose when androgens rise.
-
Psych: Mood changes/irritability in a subset.
-
Contraindications: Hormone-sensitive cancers, pregnancy (outside ART indication), active thromboembolic disease, uncontrolled endocrine disorders.
-
Drug interactions: Additive androgenic effects with AAS/TRT; aromatase inhibitors/SERMs sometimes co-used to manage estradiol/gynecomastia in men aiming at fertility.
Comparative safety matrix
| Feature | hCG | Testosterone (TRT) | FSH (uFSH/rFSH) | GnRH agonist |
|---|---|---|---|---|
| Fertility while treating hypogonadism | Preserved | Suppresses(azoospermia risk) | Supports spermatogenesis | Stimulates axis (if intact) |
| Primary target | LH receptor | Androgen receptor | FSH receptor | Pituitary GnRH receptor |
| Key risk | Gynecomastia / OHSS (women) | Erythrocytosis, infertility | Cost, injections | Flare, hypoestrogenic sx |
| Use in ART | Trigger / luteal support | No | Ovarian stimulation | Trigger (alt), down-reg |
7. Regulatory & Anti-Doping Landscape
-
Approvals: Widely approved for male HH, induction of ovulation/ART trigger, and cryptorchidism (regional differences).
-
Weight-loss warning: The “hCG diet” has been discredited; FDA/FTC warn against hCG for weight loss—no efficacy, unnecessary risk.
-
Sport: Prohibited by WADA in males (can mask or restore T after AAS) and is detectable; sanctions are common.
8. Future Directions
-
Personalized male fertility protocols: Optimizing hCG+FSH schedules by baseline testicular volume, AMH/inhibin B, and genetics.
-
Adjuncts to reduce OHSS: Wider adoption of GnRH-agonist/kisspeptin triggers and tailored luteal support instead of high-dose hCG in high responders.
-
Functional hypogonadism trials: Head-to-head hCG vs TRT (QoL, metabolic outcomes, fertility metrics).
-
Long-acting r-hCG and self-injector devices to improve adherence and cost-effectiveness.
Selected References
-
Journal of Clinical Endocrinology & Metabolism; Endocrine Reviews — Diagnosis/management of male hypogonadism and use of hCG ± FSH for fertility.
-
Fertility and Sterility; Human Reproduction Update — IVF trigger strategies (hCG vs GnRH-agonist vs kisspeptin) and OHSS mitigation.
-
Andrology; Human Reproduction — Fertility preservation during TRT using low-dose hCG; recovery after AAS.
-
Pediatrics; European Urology — Cryptorchidism medical therapy evidence and limitations.
-
Best Practice & Research Clin Endocrinol Metab — hCG stimulation tests in disorders of sex development/steroidogenesis.
-
FDA/FTC notices; Obesity — Warnings against the hCG diet and lack of weight-loss efficacy.
-
WADA / Drug Testing & Analysis — Prohibited status, detection methods, and case report