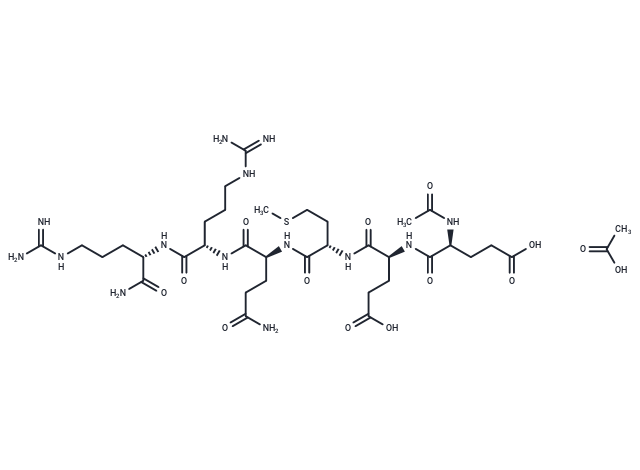
FOXO4-DRI 10mg vial
El retiro no está disponible actualmente
NOT FOR HUMAN CONSUMPTION
FOXO4-DRI (FOXO4-D-Retro-Inverso peptide) is a synthetic peptide designed as a selective senolytic agent, initially developed by researchers at Erasmus University Medical Center (Rotterdam, Netherlands). It targets senescent cells—aged cells that no longer divide but persist in the body, driving inflammation, tissue dysfunction, and aging-related diseases.
FOXO4-DRI was introduced by Baar et al. (2017) as a promising tool for selective elimination of senescent cells by disrupting the FOXO4-p53 interaction, a crucial survival mechanism used by senescent cells.
Chemical Properties and Design
-
Full Name: FOXO4-D-Retro-Inverso peptide
-
Structure: Synthetic, 26-amino-acid peptide composed entirely of D-amino acids (mirror-image stereoisomers), making it resistant to protease degradation.
-
Mechanism of Action: Specifically disrupts the interaction between the FOXO4 transcription factor and the tumor-suppressor protein p53 in senescent cells, allowing senescent cells to undergo apoptosis.
Mechanism of Action (Detailed)
Senescent cells resist apoptosis by sequestering the p53 protein in the nucleus via interaction with FOXO4, preventing p53 from initiating programmed cell death. FOXO4-DRI competitively binds FOXO4, liberating p53. This liberation triggers apoptosis specifically in senescent cells while sparing healthy cells, thereby reducing the senescent cell burden in tissues.
This selectivity is a significant advantage over nonspecific senolytics, which might harm healthy cells. Its retro-inverso configuration further enhances stability and bioavailability, making it more suitable for therapeutic applications.
Preclinical Research and Therapeutic Potential
FOXO4-DRI has shown remarkable effects across several preclinical rodent models:
1. Aging and Frailty (Baar et al., 2017)
-
Protocol: Daily injections (5 mg/kg) for five consecutive days followed by weekly injections.
-
Outcomes:
-
Improved physical function (grip strength, endurance).
-
Reduced senescent cell burden in kidney and liver.
-
Improved coat quality and skin rejuvenation.
-
2. Chemotherapy-induced Senescence (Doxorubicin Model)
-
Protocol: 5 mg/kg, every 3 days.
-
Outcomes:
-
Restoration of hematopoietic stem cell function.
-
Reduced chemotherapy-induced frailty and organ damage.
-
3. Pulmonary and Organ Fibrosis Models
-
Protocol: Subcutaneous injections (3 mg/kg every 2 days).
-
Outcomes:
-
Reduction in lung fibrosis and improved respiratory function.
-
Decreased collagen deposition and fibrotic markers.
-
4. Cancer (Experimental Models: Triple-negative Breast Cancer)
-
FOXO4-DRI derivatives (e.g., CL04177) showed potent effects on reducing tumor growth, metastases, and recurrence in mouse xenograft models by targeting chemotherapy-resistant senescent tumor cells.
Potential Therapeutic Applications
-
Senescence-associated diseases (generalized anti-aging, frailty).
-
Fibrotic diseases (pulmonary fibrosis, liver cirrhosis, renal fibrosis).
-
Chemotherapy side effects (reducing chemotherapy-induced cellular senescence).
-
Cancer therapy (targeting senescent tumor cells, particularly chemotherapy-resistant cancer cells).
-
Hormonal and reproductive aging (restoring testicular function and steroidogenesis in aging models).
Experimental Dosage (Preclinical Context)
The following dosages are extrapolated from rodent studies and represent experimental protocols:
-
Intraperitoneal (common):
-
Initial Phase: 5 mg/kg daily × 5 days.
-
Maintenance Phase: 5 mg/kg weekly thereafter.
-
-
Subcutaneous: 3 mg/kg every 48 hours (common in fibrosis models).
-
Intravenous (derivative peptides): ~1-2 mg/kg weekly (experimental oncology use).
Note: Human-equivalent doses are yet undetermined and require rigorous clinical testing.
Safety Profile and Potential Risks
Potential Safety Concerns:
-
Loss of beneficial senescence: Not all senescent cells are detrimental (some facilitate wound healing, tissue repair).
-
p53 pathway modulation: Long-term manipulation of p53 requires careful consideration due to its role in tumor suppression.
-
Immunogenicity: While D-amino acids reduce immunogenicity, repeated administrations may still trigger immune responses.
-
Unknown Long-Term Risks: No extensive long-term studies to evaluate chronic use, potential carcinogenic risk, or systemic effects.
Reported Side Effects (Rodent studies):
-
Mild, transient weight loss or lethargy at initiation.
-
No major toxicity noted short-term, but chronic administration and long-term safety remain largely unstudied.
Regulatory Status and Developmental Pipeline
As of April 2025:
-
FOXO4-DRI remains an experimental, research-only compound.
-
Cleara Biotech (Netherlands), the company formed by the original FOXO4-DRI inventors, is currently optimizing second-generation derivatives (e.g., CL04177, CL04183) for improved pharmacokinetics, selectivity, and therapeutic window.
-
No formal clinical trials (Phase I or later) have been registered or initiated, though Cleara indicates plans for Phase I oncology trials within the next 18 months.
-
Current status: IND-enabling studies are underway for derivatives targeting specific oncology indications.
Key Limitations and Research Gaps
-
Lack of human clinical data: All available efficacy and safety data derive solely from animal studies.
-
Optimal dosing strategy: Frequency, duration, and appropriate administration routes in humans remain uncharacterized.
-
Long-term safety: No chronic toxicity, immunogenicity, or cancer-risk assessment in long-term human trials.
-
Biomarker development: Needed to reliably distinguish harmful vs. beneficial senescent cell subpopulations.
Future Directions
-
First-in-human trials: Critical for determining safety, pharmacokinetics, and therapeutic efficacy.
-
Development of improved derivatives: Cleara Biotech’s next-generation peptides promise greater selectivity and bioavailability, potentially addressing earlier safety concerns.
-
Combination therapies: Potential synergy with other senolytics, chemotherapeutics, or regenerative medicine approaches.
Conclusion and Current Outlook
FOXO4-DRI represents a pioneering concept in the rapidly growing field of senolytics, targeting a fundamental mechanism underlying cellular senescence. Preclinical research has provided compelling evidence of its potential to treat age-related diseases, fibrosis, cancer recurrence, and chemotherapy-induced damage. However, no human clinical trials have yet been conducted, and significant safety and efficacy questions remain. As such, FOXO4-DRI currently remains an exciting, yet strictly experimental peptide awaiting clinical validation.
References
-
Baar MP et al. (2017). "Targeted apoptosis of senescent cells restores tissue homeostasis." Cell, 169(1), 132-147.
-
Cleara Biotech official announcements (2022-2025): Senolytic pipeline updates and derivatives (CL04177, CL04183).
-
Croker JA et al. (2024). "Therapy-induced senescence: FOXO4-DRI as a targeted senolytic approach." Trends Pharmacol Science.
Disclaimer:
This review is provided solely for educational purposes. FOXO4-DRI remains an experimental compound without regulatory approval for human use. Always consult qualified healthcare providers before considering any experimental treatments or interventions.